Abstract
Introduction
Individuals with sickle cell disease (SCD) in resource-rich countries are now living longer due to the implementation of newborn screening, use of prophylactic penicillin, and introduction of hydroxyurea. The impact of SCD on the bone marrow and peripheral blood counts as individuals with SCD age is not well described in the hydroxyurea era. As a result of increased red blood cell (RBC) turnover due to hemolysis in SCD, potential ineffective erythropoiesis, and well-described systemic inflammatory processes, the increased stress in the bone marrow may manifest as decreased peripheral blood cell counts over time in a during an aging process unique to those with SCD.
Objective
The objective was to model routine clinical blood measurements (n=5,328) with a longitudinal cohort of 447 patients, resulting in models of circulating blood counts as a potential marker of bone marrow function across the lifespan in adults with SCD. Our working hypothesis was that increasing age would be associated with decreases in certain blood counts, with trends modified by SCD genotype severity.
Methods
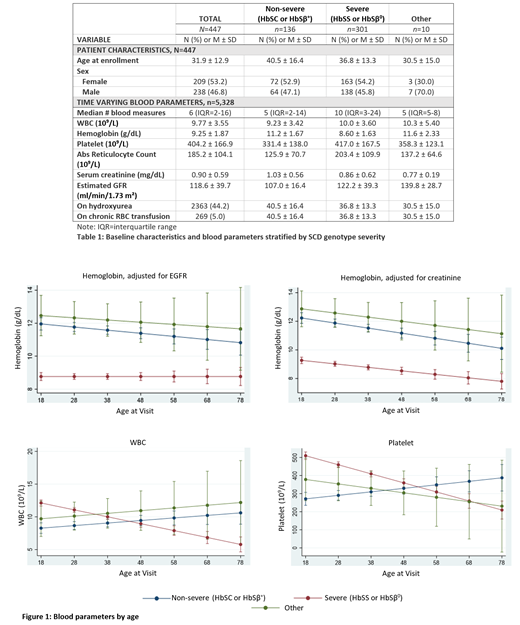
In this large, single institution retrospective cohort study, we analyzed blood count measurements from adults with SCD. We used an accelerated longitudinal cohort design to examine changes in white blood cell count (WBC), hemoglobin (HGB), absolute reticulocyte count (ARC), and platelet count over time. Genotypes were grouped as severe (HbSS or HbSβ 0-thalassemia; n=301), non-severe (HbSC or HbSβ +-thalassemia; n=136), or other (n=10). For HGB and ARC, we adjusted for estimated glomerular filtration rate (eGFR) and serum creatinine.
Results
Patients ranged in age at enrollment from 18 to 84 (M = 31.9, SD=12.9), with median follow-up time of 3.6 years and a median of 6 measurements per patient. Overall, HGB and ARC decreased across the lifespan; however, trends were moderated by genotype. Compared to individuals with non-severe genotypes, those with the severe genotypes had consistently lower HGB levels that did not differ by age. For ARC, the severe genotypes had higher levels in young adulthood, which slowly decreased with age. WBC also decreased with age for this group. In contrast, individuals with the non-severe genotype showed an increase in WBC by age. Platelet counts decreased with increasing age for those with the severe genotype while those with non-severe genotypes showed an increase with age.
Conclusion
Severe SCD genotypes are associated with decreases in HGB, ARC, WBC, and platelet count by age. For HGB and ARC, these declines are significantly attenuated by renal function, suggesting that such declines are largely related to worsening renal function with age. However, an intrinsic bone marrow contribution to this aging process should also be explored in future studies. Decreases in WBC and platelet count with time, observed in the severe genotypes, support the presence of pathological processes of bone marrow dysfunction that may worsen with age. A possible etiology of the increasing bone marrow dysfunction in SCD may be a result of bone marrow exhaustion or cellular senescence. Prospective studies to explore contributors to reduced blood counts during the aging process in individuals with SCD are needed. We are actively exploring potential biomarkers associated with this process.
Carden: Forma Therapeutics: Current Employment. Derebail: Novartis: Consultancy; UpToDate: Patents & Royalties; Travere Therapeutics: Consultancy; Bayer: Consultancy. Ataga: Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Consultancy; Forma Therapeutics: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees.